The binding mode of vilazodone in the human serotonin transporter elucidated by ligand docking and molecular dynamics simulations†
Abstract
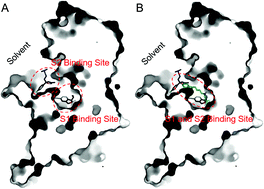
Vilazodone is a novel antidepressant used for the treatment of major depressive disorder (MDD) with a primary action mechanism of inhibiting the human serotonin reuptake transporter (hSERT) and acting as a 5-HT1A receptor partial agonist. The interaction between vilazodone and the 5-HT1A receptor has been reported, however, the binding mode of vilazodone in the hSERT remains elusive. In the current study, to elucidate the molecular mechanism of vilazodone binding in the hSERT, the drug and its five analogs were docked into the hSERT crystal structure as initial conformations and were sampled by 400 ns molecular dynamics (MD) simulations. Through the analysis of the profiles of protein–ligand binding free energies, interaction fingerprints, and conformational rearrangements, the binding mode of vilazodone in the hSERT was revealed. As a result, unlike the classical antidepressants located in the S1 site of the hSERT, vilazodone adopted a linear pose in the binding pocket. Its arylpiperazine fragment occupies the central site (S1) and interacts with Y95, D98, I172, Y176, F335, F341, S438, and T439, while the indole fragment extends to the allosteric site (S2) via interacting with the ionic switch (R104/E403) between the two sites. The new insights obtained are not only helpful in understanding the binding mode of vilazodone in the hSERT, but also provide valuable guidance to the discovery of novel antidepressant drugs.


 Please wait while we load your content...
Please wait while we load your content...