Desorption products during linear heating of copper zeolites with pre-adsorbed methanol†
Abstract
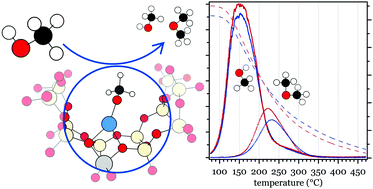
Desorption products from zeolites with medium (MFI) and small (CHA) pores and with and without ion-exchanged copper were studied during linear heating after the pre-adsorption of methanol using a chemical flow reactor with a gas phase Fourier transform infrared spectrometer. The methanol desorption profiles were deconvoluted and compared with those predicted from first-principles calculations. In situ diffuse reflectance infrared Fourier transform spectroscopy was used to study the samples during methanol desorption following a step-wise increase of the sample temperature. It is shown that well-dispersed copper species in the Cu-zeolite samples interact more strongly with methanol and its derivatives as compared to the bare zeolites, resulting in methanol desorption at higher temperatures. Moreover, the introduction of Cu leads to CO formation and desorption in larger amounts at lower temperatures compared to the bare zeolites. The formation and desorption of dimethyl ether (DME) from pre-adsorbed methanol takes place at different temperatures depending on both the influence of Cu and the zeolite topology. The Cu sites in zeolites lead to higher DME formation/desorption temperatures, while a small shift of DME desorption towards higher temperatures is observed for the CHA framework structure compared to the MFI framework structure.


 Please wait while we load your content...
Please wait while we load your content...