Attack of hydroxyl radicals to α-methyl-styrene sulfonate polymers and cerium-mediated repair via radical cations†
Abstract
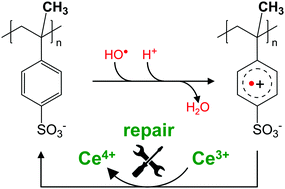
Both synthetic polymers (membranes, coatings, packaging) and natural polymers (DNA, proteins) are subject to radical-initiated degradation. In order to mitigate the deterioration of the polymer properties, antioxidant strategies need to be devised. We studied the reactions of poly(α-methylstyrene sulfonate), a model compound for fuel cell membrane materials, with different degrees of polymerization with OH˙ radicals as well as subsequent reactions. We observed the resulting OH˙-adducts to react with oxygen and eliminate H2O, the relative likelihood of which is determined by pH and molecular weight. The resulting radical cations can be reduced back to the parent molecule by cerium(III). This ‘repair’ reaction is also dependent on molecular weight likely because of intramolecular stabilization. The results from this study provide a starting point for the development of new hydrocarbon-based ionomer materials for fuel cells that are more resistant to radical induced degradation through the detoxification of intermediates via damage transfer and repair pathways. Furthermore, a more fundamental understanding of the mechanisms behind conventional antioxidants in medicine, such as ceria nanoparticles, is achieved.


 Please wait while we load your content...
Please wait while we load your content...