The effect of surface polarity on the structure and collective dynamics of liquid ethanol†
Abstract
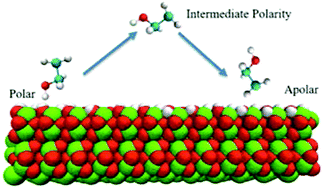
We have performed molecular dynamics simulations to study the structure and dynamics of liquid ethanol at the α-Al2O3 surface. We scale the surface charges by a dimensionless parameter k, ranging from 0 to 1, which allows us to model the surface changing from apolar to polar. Compared with previous studies on hydrophilic surfaces, interfacial ethanol molecules form less densely packed layering structures and show less preferential orientational ordering as the alumina surface gradually becomes more apolar. Further analysis of hydrogen bonds and density correlation functions suggest that both hydrogen bonding between ethanol and the alumina surface and the interdigitation of alkyl groups control the interfacial structures collectively. Rotational dynamics and in-plane diffusion analysis show an acceleration of interfacial dynamics as the surface is switched to nonpolar, whereas interfacial rotational dynamics does not follow a monotonic trend during polarity tuning. Extending in-plane diffusion analysis to the second layer of the interface shows a strong enhancement of diffusion from the first layer at the polar interface in comparison to the apolar surfaces, suggesting different surface diffusion mechanisms at polar surfaces that may play an important role in the mobile phase transport in liquid chromatography.


 Please wait while we load your content...
Please wait while we load your content...