The effect of coordination environment on the kinetic and thermodynamic stability of single-atom iron catalysts†
Abstract
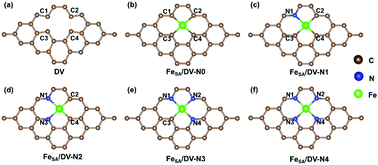
The stability of a single-atom catalyst is directly related to its preparation and applications, especially for high-loading single-atom catalysts. Here, the effect of a coordination environment induced by nitrogen (N) atoms coordinated with iron on the kinetic and thermodynamic stabilities of single-atom iron catalysts supported with carbon-based substrates (FeSA/CS) was investigated by density functional theory (DFT) calculations. Five FeSA/CS with different numbers of N atoms were modelled. The kinetic stability was evaluated by analyzing the migration paths of iron atoms and energy barriers. The thermodynamic stability was studied by calculating the adsorption and formation energies. Our results indicated that the coordination environment induced by N can promote the kinetic and thermodynamic stability of FeSA/CS. N atoms on the substrate promote the kinetic stability by raising the energy barrier for iron migration and not only increase the thermodynamic stability, but also contribute to catalyst synthesis. Doping N on the substrate enhances charge transfer between the iron atoms and substrates simultaneously improving the kinetic and thermodynamic stabilities. This theoretical research provides guidance for synthesizing stable and high loading single-atom catalysts by tuning the coordination environment of single-atom elements.


 Please wait while we load your content...
Please wait while we load your content...