Dissociative photodetachment dynamics of the oxalate monoanion†
Abstract
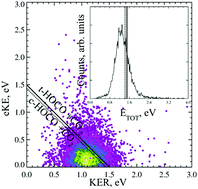
The dissociative photodetachment (DPD) dynamics of the oxalate monoanion are studied using photoelectron–photofragment coincidence (PPC) spectroscopy. Following photodetachment of C2O4H− at 4.66 eV HOCO + CO2 products are observed, indicating the facile decarboxylation of the radical driven by the thermodynamic stability of CO2. No evidence is seen for photodetachment to stable C2O4H or ionic photodissociation to produce HOCO−. Calculations indicate the stabilizing presence of an intramolecular hydrogen bond in the anion via the formation of a strained five-membered ring. No intramolecular hydrogen bond is predicted in the radical due to the lower charge density on the oxygen atom. The PPC spectrum is consistent with a single direct two-body DPD channel that results in fragments of similar mass and is characterized by a large kinetic energy release (KER) and a broad photoelectron spectrum. The large KER is indicative of substantial repulsion in the radical following photodetachment. The form of the photoelectron spectrum is dominated by the bound to continuum Franck–Condon factors (BCFCF) and is suggestive of photodetachment to a repulsive potential energy surface. A lower bound for the electron affinity of C2O4H is reported as 4 eV. BCFCF calculations allow an approximate functional form of the repulsive surface along the C–C stretch coordinate to be extracted from the experimental photoelectron spectrum. PPC spectroscopy of the deuterated analogue (C2O4D−), at higher anion beam energies is used to increase the detectability of any possible D atom products, but none are observed.


 Please wait while we load your content...
Please wait while we load your content...
