Targeting the heme protein hemoglobin by (−)-epigallocatechin gallate and the study of polyphenol–protein association using multi-spectroscopic and computational methods†
Abstract
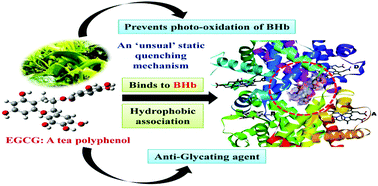
In this work, the interaction of a bioactive tea polyphenol (−)-epigallocatechin gallate (EGCG) with bovine hemoglobin (BHb) along with its anti-oxidative behavior and the anti-glycation property have been explored using multi-spectroscopic and computational techniques. The binding affinity for EGCG towards BHb was observed to be moderate in nature with an order of 104 M−1, and the fluorescence quenching mechanism was characterized by an unusual static quenching mechanism. The binding constant (Kb) showed a continuous enhancement with temperature from 3.468 ± 0.380 × 104 M−1 at 288 K to 6.017 ± 0.601 × 104 M−1 at 310 K. The fluorescence emission measurements along with molecular docking studies indicated that EGCG binds near the most dominant fluorophore of BHb (β2-Trp37, at the interface of α1 and β2 chains) within the pocket formed by the α1, α2 and β2 chains. The sign and magnitude of the thermodynamic parameters, changes in enthalpy (ΔH = +17.004 ± 1.007 kJ mol−1) and in entropy (ΔS = +146.213 ± 2.390 J K−1 mol−1), indicate that hydrophobic forces play a major role in stabilizing the BHb–EGCG complex. The micro-environment around the EGCG binding site showed an increase in hydrophobicity upon ligand binding. The binding of EGCG with BHb leads to a decrease in the α-helical content, whereas that of the β-sheet increased. FTIR studies also indicated that the secondary structure of BHb changed upon binding with EGCG, along with providing further support for the presence of hydrophobic forces in the complexation process. Molecular docking studies indicated that EGCG binds within the cavity of α1, α2, and β2 chains surrounded by residues such as α1- Lys99, α1-Thr134, α1-Thr137, α1-Tyr140, α2-Lys127 and β2-Trp37. Molecular dynamics simulation studies indicated that EGCG conferred additional stability to BHb. Furthermore, moving away from the binding studies, EGCG was found to prevent the glyoxal (GO)-mediated glycation process of BHb, and it was also found to act as a potent antioxidant against the photo-oxidative damage of BHb.


 Please wait while we load your content...
Please wait while we load your content...