An electric double layer structure and differential capacitance at the electrode interface of tributylmethylammonium bis(trifluoromethanesulfonyl)amide studied using a molecular dynamics simulation†
Abstract
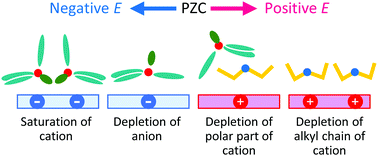
A molecular dynamics simulation at the electrode interface of a quaternary ammonium ionic liquid, tributylmethylammonium bis(trifluoromethanesulfonyl)amide ([N1444+][TFSA−]), has been performed. Unlike the commonly used cations, such as 1-alkyl-3-methylimidazolium and 1,1-alkylmethylpyrrolidinium cations, N1444+ has multiple long-alkyl groups (three butyl groups). The behavior of ions at the electrode interface, especially these butyl groups, has been investigated. N1444+ at the first layer mainly has two types of orientations, lying and standing. The lying orientation is dominant at moderately negative potentials. However, the standing one becomes dominant at the more negative potentials. Due to this orientational change, the number of N1444+ increases at the first layer as the potential becomes negative even at the potentials where the anions are completely depleted there. The change in orientation results in the upward deviation of the differential capacitance from the theoretical prediction at the negative potentials. The results suggest that the orientational preference caused by the steric constraint between alkyl groups plays an important role in the behavior of the electric double layer of the ionic liquids.


 Please wait while we load your content...
Please wait while we load your content...