Compressive-force induced activation of apo-calmodulin in protein signalling†
Abstract
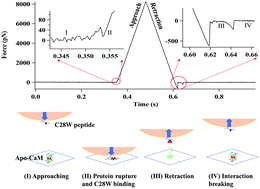
Mechanical force plays a critical role in the relationship between protein structure and function. Force manipulation by Atomic Force Microscope can be significant and trigger chemical and biological activities of proteins. Previously we have reported that Apo-CaM undergoes through a spontaneous tertiary structural rupture under a piconewton compressive force. Here we have observed that the ruptured Apo-CaM molecules can be available to bind with C28W peptide, a typical protein signalling activity that only a Ca2+-activated CaM has. This behaviour is both unexpected and profound, as CaM in its Ca2+-non-activated form has a closed structure which does not presumably allow the molecule to bind to target peptides. In this experiment, we demonstrate that both chemical activation and force activation can play a vital role in biology, such as the cell-signalling protein dynamics and function.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...