Influence of residual water and cation acidity on the ionic transport mechanism in proton-conducting ionic liquids
Abstract
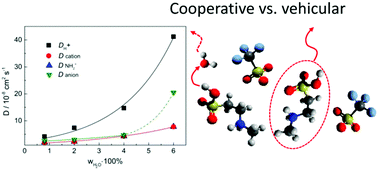
Proton-conducting ionic liquids (PILs) are discussed herein as potential new electrolytes for polymer membrane fuel cells, suitable for operation temperatures above 100 °C. During fuel cell operation, the presence of significant amounts of residual water is unavoidable, even at these elevated temperatures. By using electrochemical and NMR methods, the impact of residual water on 2-sulfoethylmethylammonium triflate [2-Sema][TfO], 1-ethylimidazolium triflate [1-EIm][TfO] and diethylmethylammonium triflate [Dema][TfO] is analyzed. The cationic acidity of these PILs varies by over ten orders of magnitude. Appropriate amounts of the PIL and H2O were mixed at various molar ratios to obtain compositions, varying from the neat PIL to H2O-excess conditions. The conductivity of [2-Sema][TfO] exponentially increases depending on the H2O concentration. The results from 1H-NMR spectroscopy and self-diffusion coefficient measurements by 1H field-gradient NMR indicate a fast proton exchange process between [2-Sema]+ and H2O. Conversely, [1-EIm][TfO] and [Dema][TfO] show only very slow or non-significant proton exchange, respectively, with H2O during the time-scale relevant for transport. The proton conduction follows a combination of vehicle and cooperative mechanisms in highly acidic PIL, while a mostly vehicle mechanism in medium and low acidic PIL occurs. Therefore, highly acidic ionic liquids are promising new candidates for polymer electrolyte fuel cells at an elevated temperature.


 Please wait while we load your content...
Please wait while we load your content...