Effect of ammonia and formic acid on the CH3O˙ + O2 reaction: a quantum chemical investigation†
Abstract
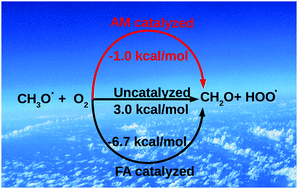
In the present work, the catalytic effect of ammonia and formic acid on the CH3O˙ + O2 reaction has been investigated employing the MN15L density functional. The investigations suggest that, in the presence of ammonia, the reaction can proceed through two different pathways, namely a single hydrogen atom transfer and a double hydrogen atom transfer path, but due to the high energy barrier associated with the double hydrogen atom transfer channel, it prefers the single hydrogen atom transfer channel. On the other hand, in the case of formic acid, only the single hydrogen atom transfer path is found to be feasible. Interestingly, it has been found that, in the presence of ammonia and formic acid, the reaction becomes a barrierless reaction. The calculated rate constant values at various temperatures indicate an anti-Arrhenius behavior for both the ammonia and formic acid catalyzed channels.


 Please wait while we load your content...
Please wait while we load your content...