Interactions of ions across carbon nanotubes
Abstract
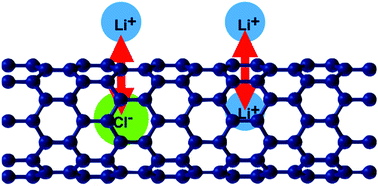
The interactions between a pair of Li+ ions across a semiconducting (8,0)CNT and a conducting (5,5)CNT has been investigated by density functional theory. The direct Coulomb interaction between the ions is almost completely screened. The band structure of the CNTs is not affected by the Li+ ions, but the Fermi level is raised to accommodate the extra electrons. Because of the unique band structure of CNTs this results in an effective attraction between the ions, which is greater for the (8,0)CNT. In contrast, a Cl− ion inside a CNT forms a chemical bond which modifies the band structure. Again, the electrostatic field of the ion is almost completely screened outside of the tube. Nevertheless, the adsorption of a Li+ ion outside is favored by a Cl− ion inside. This apparent attraction is mainly caused by a lowering of the work function of the CNT by the presence of the Cl−.
- This article is part of the themed collection: Frontiers in Molecular Simulation of Solvated Ions, Molecules and Interfaces


 Please wait while we load your content...
Please wait while we load your content...