In situ7Li-NMR analysis of lithium metal surface deposits with varying electrolyte compositions and concentrations†
Abstract
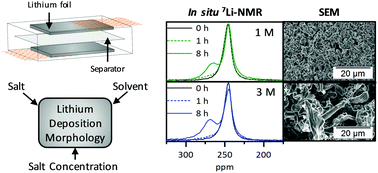
A major challenge of lithium metal electrodes, in theory a suitable choice for rechargeable high energy density batteries, comprises non-homogeneous lithium deposition and the growth of reactive high surface area lithium, which eventually yields active material losses and safety risks. While it is hard to fully avoid inhomogeneous deposits, the achievable morphology of the occurring lithium deposits critically determines the long-term cycling behaviour of the cells. In this work, we focus on a combined scanning electron microscopy (SEM) and 7Li nuclear magnetic resonance spectroscopy (7Li-NMR) study to unravel the impact of the choice of conducting salts (LiPF6 and LiTFSI), solvents (EC : DEC, 3 : 7, DME : DOL, 1 : 1), as well as their respective concentrations (1 M, 3 M) on the electrodeposition process, demonstrating that lithium deposition morphologies may be controlled to a large extent by proper choice of cycling conditions and electrolyte constituents. In addition, the applicability of 7Li-NMR spectroscopy to assess the resulting morphology is discussed. It was found, that lithium deposition analysis based on the 7Li chemical shift and intensity should be used carefully, as various morphologies can lead to similar results. Still, our case study reveals that the combination of SEM and NMR data is rather advantageous and offers complementary insights that may provide pathways for the future design of tailored electrolytes.


 Please wait while we load your content...
Please wait while we load your content...