Reactive mode composition factor analysis of transition states: the case of coupled electron–proton transfers†
Abstract
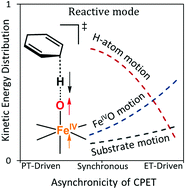
A simple method for the evaluation of the kinetic energy distribution within the reactive mode of a transition state (TS), denoted as the Reactive Mode Composition Factor (RMCF), is presented. It allows one to directly map the barrier properties onto the atomic-motion components of the reaction coordinate at the TS, which has potential to shed light onto some mechanistic features of a chemical process. To demonstrate the applicability of RMCF to reactivity, we link the kinetic energy distribution within a reactive mode with the asynchronicity (η) in C–H bond activation, as they both evolve in a series of coupled proton–electron transfer (CPET) reactions between FeIVO oxidants and 1,4-cyclohexadiene. RMCF shows how the earliness or lateness of a process manifests as a redistribution of kinetic energy in the reactive mode as a function of the free energy of reaction (ΔG0) and η. Finally, the title analysis can be applied to predict H-atom tunneling contributions and kinetic isotope effects in a set of reactions, yielding a transparent rationalization based on the kinetic energy distributions in the reactive mode.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...