Magnesium oxide clusters as promising candidates for hydrogen storage†
Abstract
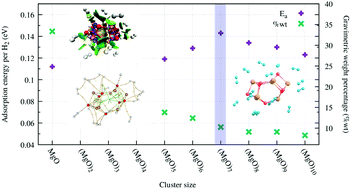
With the idea of proposing solid state systems that have a high storage capacity of molecular hydrogen, a density functional theory study of magnesium oxide (MgO)n clusters (n = 1–10) was carried out. Hydrogen–magnesium oxide systems presented adsorption energy values in accordance with the previously reported studies of physisorption processes; additionally negative values of ΔGads were found describing adsorption as a favorable process. Here, the (MgO)7 cluster presented the highest adsorption energy. The storage capacity by weight of the magnesium oxide clusters was greater than the recommended percentage (7.5%) by the U.S. Department of Energy. QTAIM analysis and non-covalent index plots highlighted the weak nature of the interaction between the MgO clusters and hydrogen molecules, and the fundamental role of the Mg–O bonds’ polarity in the systems’ storage capacity.


 Please wait while we load your content...
Please wait while we load your content...