Photoswitching hydrazones based on benzoylpyridine†
Abstract
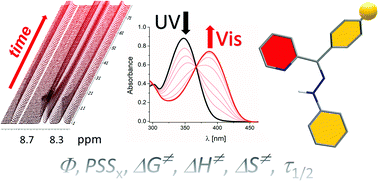
The photoswitching properties of three readily accesible benzoylpyridine hydrazones were investigated. Interestingly, replacing classical stirring with ultrasound wave activation results in pure thermodynamically less stable E isomer crystallization at room temperature. The studied benzoylpyridine hydrazones exhibit both P- and T-photochromic behaviour, depending on the benzoyl substituent, and improved addressability compared to most of the previously published pyridyl based hydrazones and 2-pyridylcarboxaldehyde acylhydrazones. Low activation entropy and calculated transition state geometry favour the inversion mechanism of their thermal isomerization rather than tautomerization followed by rotation recently found for pyridyl-hydrazone ester or nitrile rotary switches. The association behaviour of the nitro derivative during its thermal E-to-Z isomerization in highly polar DMSO indicates an important role of intermolecular hydrogen bonding in the thermal kinetics of benzoylpyridine-based hydrazone photoswitches. Moreover, the addition of triethylamine significantly accelerates the rate of Z-isomer thermal isomerization from days to seconds and could thus pave the way to fast pyridyl hydrazone T-type photochromic compounds in polar solvents. This study could therefore contribute to general knowledge related to the photochromic behaviour of hydrazones as an important class of modern photoswitches.


 Please wait while we load your content...
Please wait while we load your content...