Atmospheric loss of nitrous oxide (N2O) is not influenced by its potential reactions with OH and NO3 radicals†
Abstract
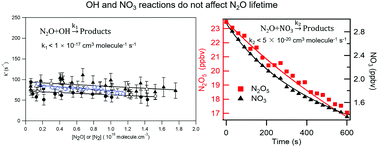
The rate coefficient for the possible reaction of OH radical with N2O was determined to be k1 < 1 × 10−17 cm3 molecule−1 s−1 between 253 and 372 K using pulsed laser photolysis to generate OH radicals and pulsed laser induced fluorescence to detect them. The rate coefficient for the reaction of NO3 radical with N2O was measured to be k2 < 5 × 10−20 cm3 molecule−1 s−1 at 298 K using a direct method that involves a large reaction chamber equipped with cavity ring down spectroscopic detection of NO3 and N2O5. Various tests were carried out ensure the accuracy of our measurements. Based on our measured upper limits, we suggest that these two reactions alter the atmospheric lifetime of N2O of ∼120 years by less than 4%.


 Please wait while we load your content...
Please wait while we load your content...