The doping and oxidation of 2D black and blue phosphorene: a new photocatalyst for nitrogen reduction driven by visible light†
Abstract
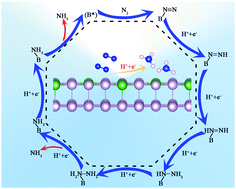
The synthesis of NH3 under ambient conditions using solar energy is the most attractive method of synthesis, and requires efficient and stable photo-catalysts with ultralow overpotentials, because a low overpotential ensures low energy consumption and a high yield of NH3. Here, by using density functional theory calculations, the catalytic activity enhancements from B decoration and the oxidation of black and blue phosphorene for N2 fixation photo-catalysis are predicted. The results show that the computed overpotentials (ηNRR) were 0.78 and 0.92 V through the end-on configuration of the B doped black phosphorene (B-BlP) and blue phosphorene (B-BeP), respectively. In addition, owing to the reduction of the band gap, decoration with B can greatly enhance the visible light and infrared light harvesting of BlP and BeP, guaranteeing them as ideal potential materials for the visible light reduction of N2. The formation energy of B decoration and the ab initio molecular dynamics simulation results indicate that B-BlP and B-BeP are highly stable under ambient conditions (300 K). The oxidation of the BlP and BeP surfaces can also enhance the N2 reduction reaction (NRR) performances using visible light driving, with the ηNRR values being 1.31 and 1.51 V through the end-on and side-on configurations, respectively. In summary, this work offers the opportunity for decorated phosphorene to act as a photo-catalyst for NH3 production.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...