Rapid carbene formation increases ion diffusivity in an imidazolium acetate ionic liquid confined between polar glass plates
Abstract
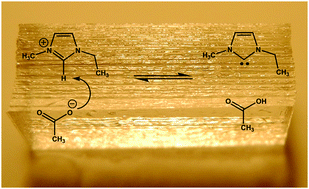
1-Ethyl-3-methyl-imidazolium acetate ([EMIM][OAc]) is one of the most widely used ionic liquids for various applications. This study is focussed on the chemical stability of [EMIM][OAc] on the surfaces of polar glass plates. 1H and 13C NMR spectroscopy and NMR diffusometry of [EMIM][OAc] IL confined between glass plates with a specific surface area 105–106 m−1 are thoroughly investigated. A rapid and spontaneous reaction took place on the surfaces of glass plates leading to the formation of neutral chemical moieties as evident by the appearance of new signals in the 1H NMR spectra. These new products are assigned as N-heterocyclic carbene (NHC) and acetic acid. These neutral chemical moieties have significantly increased the ion diffusivity by dissociation of the cation and the anion in [EMIM][OAc] IL. The yield and rate of formation of NHC and acetic acid are found to increase with the increasing surface area of polar glass plates and the time of contact between the IL and glass surfaces. Based on NMR spectroscopy, a dissociative reaction mechanism is proposed for the formation of free NHC in the neat [EMIM][OAc] IL.


 Please wait while we load your content...
Please wait while we load your content...