UV-promoted radical formation, and near-IR-induced and spontaneous conformational isomerization in monomeric 9-methylguanine isolated in low-temperature Ar matrices†
Abstract
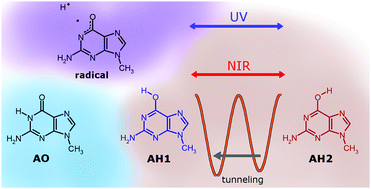
Three low-energy isomers of 9-methylguanine, the amino-oxo (AO) form and two amino-hydroxy (AH1 and AH2) conformers, were trapped from the gas phase into low-temperature argon matrices. The AH1 and AH2 isomers, differing in the orientation of the OH group, were found to transform into each other upon excitation with near-IR light. The population of the AO form of the compound was not changed upon any near-IR irradiation of the matrix samples. Using monochromatic near-IR light, generated by a frequency-tunable laser source, it was possible to selectively induce the AH1 → AH2 or AH2 → AH1 conversion. Photoreversibility of this conformational transformation was then demonstrated. Exposure of matrix-isolated monomers of 9-methylguanine to broadband near-IR light also led to conformational conversions within the amino-hydroxy tautomeric form; the final stage of this process was always the same photostationary state independent of the initial ratio of AH1 and AH2 populations. Spontaneous conformational conversion, transforming the higher-energy AH2 form into the lower-energy AH1 isomer, was observed for matrix-isolated monomers of 9-methylguanine kept in the dark. The mechanism of this process must rely on quantum tunneling of the light hydrogen atom. Irradiation of matrix-isolated 9-methylguanine with UV laser light at λ = 288 or 285 nm led to a substantial consumption of the two AH forms, while the amount of AO isomer remained unchanged. On the other hand, a decrease in the population of the AO isomer occurred upon excitations at shorter wavelengths, λ = 280 or 275 nm. The spectral changes observed after UV-irradiation suggest the generation (and stabilization in the matrix) of a radical species, resulting from the photocleavage of the O–H or N1–H bonds, in the AH or AO isomer, respectively.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...