Isolating the role of hydrogen bonding in hydroxyl-functionalized ionic liquids by means of vaporization enthalpies, infrared spectroscopy and molecular dynamics simulations†
Abstract
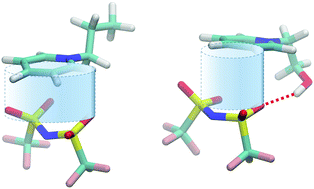
The enthalpy of vaporization is mainly the amount of the energy needed for transferring quantities from the liquid into the gas phase. It simply describes the energy required to overcome the interaction energy between quantities if those evaporate as monomers as is the case for molecular liquids. The situation for ionic liquids (ILs) is more complex. We do not know the delicate composition of different types of interaction, neither for the liquid nor for the gas phase. Additionally, we have to consider that ILs evaporate as ion pairs which carry substantial interaction energy of all kind into the vapor phase. In this study, we measured the vaporization enthalpies of well-selected hydroxyl-functionalized and non-hydroxyfunctionalized ILs. In particular, we focussed on the case of hydroxyl-functionalized ILs providing possible hydrogen bonding between cation and anion in the liquid as well as in the gas phase. With infrared spectroscopy, we showed that all the hydroxyl groups are involved in hydrogen bonding in the liquid state of the ILs. However, molecular dynamics simulations showed that the evaporating ion pairs also include this hydrogen bond. A detailed analysis of the potential energies for all IL constituents showed that the hydrogen bond hinders favourable interaction between the polarizable ring of the cations and the anions leading to higher vaporization enthalpies for the hydroxyl-functionalized ILs.


 Please wait while we load your content...
Please wait while we load your content...