Broadband rotational spectroscopy of trans 3-pentenenitrile and 4-pentenenitrile†
Abstract
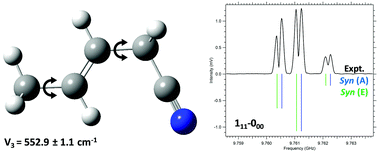
Titan, a moon of Saturn, has a nitrogen- and methane-rich atmosphere that is similar to prebiotic earth, and is replete with organic nitriles. Pentenenitriles have not yet been detected in Titan's atmosphere or in molecular clouds, but are potential precursors to hetero-aromatic compounds such as pyridine. We performed broadband microwave studies in the 8–18 GHz range on the trans isomer of 3-pentenenitrile (3-PN) and 4-pentenenitrile (4-PN) under jet-cooled conditions. Strong-field coherence breaking (SFCB) was used to selectively modulate the intensities of microwave transitions in a conformer-specific manner for 3-PN, aiding analysis. Two conformers of 3-PN and five conformers of 4-PN were identified and the rotational transitions were assigned. Evidence for methyl internal rotation splitting was observed for both the conformers of 3-PN, and the barrier heights of both conformers was determined experimentally. Comparison is made of the conformational preferences, stability and isomerization barriers through the acquired rotational spectra and potential energy surface (PES) calculations of the structural isomers 3-PN and 4-PN.


 Please wait while we load your content...
Please wait while we load your content...
