Identification of stoichiometric and microstructural parameters of a lithium-ion cell with blend electrode
Abstract
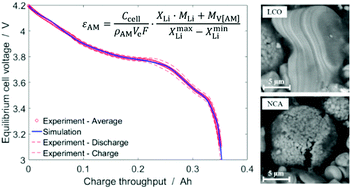
The measurement of the active material volume fraction in composite electrodes of lithium-ion battery cells is difficult due to the small (sub-micrometer) and irregular structure and multi-component composition of the electrodes, particularly in the case of blend electrodes. State-of-the-art experimental methods such as focused ion beam/scanning electron microscopy (FIB/SEM) and subsequent image analysis require expensive equipment and significant expertise. We present here a simple method for identifying active material volume fractions in single-material and blend electrodes, based on the comparison of experimental equilibrium cell voltage curve (open-circuit voltage as function of charge throughput) with active material half-cell potential curves (half-cell potential as function of lithium stoichiometry). The method requires only (i) low-current cycling data of full cells, (ii) cell opening for measurement of electrode thickness and active electrode area, and (iii) literature half-cell potentials of the active materials. Mathematical optimization is used to identify volume fractions and lithium stoichiometry ranges in which the active materials are cycled. The method is particularly useful for model parameterization of either physicochemical (e.g., pseudo-two-dimensional) models or equivalent circuit models, as it yields a self-consistent set of stoichiometric and structural parameters. The method is demonstrated using a commercial LCO–NCA/graphite pouch cell with blend cathode, but can also be applied to other blends (e.g., graphite–silicon anode).


 Please wait while we load your content...
Please wait while we load your content...