The effect of CO2 loading on alkanolamine absorbents in aqueous solutions†
Abstract
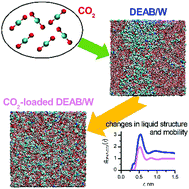
Post-combustion carbon capture by amine scrubbing is the most frequently used process to remove CO2 from pulverized coal-fired power plants and also biogas flue gas streams. The quest for novel absorbents for CO2 capture with improved properties requires insight into the properties of the CO2-loaded mixed solutions. A comparative molecular dynamics study of the product state solutions, with chemically-bound CO2 of standard monoethanolamine (MEA) and the new alternative 4-diethylamino-2-butanol (DEAB) at various CO2-loadings yields solvent properties in good agreement with experimental data. The concentration of all post-reaction species in solution was based on experimental equilibria distributions. The data generated provide detailed insight into the properties of reactive mixed alkanolamine solutions. The liquid structure of aqueous MEA solutions undergoes only minor changes when absorbing CO2. The diffusion coefficients of all molecular species, however, decrease significantly with increasing CO2-loadings. The large hydrophobic clusters formed in the reactant state by DEAB molecules in water prior to CO2 binding significantly decrease in size and structure upon CO2 absorption. The diffusion coefficients of all components decrease with increasing CO2-loading, whereas the pre-reaction alkanolamine DEAB shows an increase in diffusion coefficient. This structural and kinetic information supports the molecular design and further development of novel compounds and provides data for a global process simulation and optimization.


 Please wait while we load your content...
Please wait while we load your content...