The riddle of the forbidden UV absorption of aqueous nitrate: the oscillator strength of the n → π* transition in NO3− including second order vibronic coupling†
Abstract
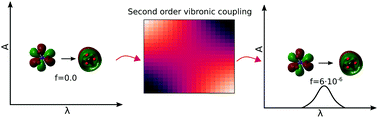
The environmentally relevant n → π* transition in the nitrate anion is doubly forbidden by symmetry, as the Franck–Condon and first order vibronic coupling terms are both zero in the gas phase. Inclusion of the second order vibronic coupling term is therefore essential when calculating the oscillator strength. Here we have calculated an oscillator strength of 5.7 × 10−6. The second order vibronic coupling term is included by manually displacing the ground-state geometry simultaneously along two normal modes, Ql and Qk, in 19 × 19 steps, and calculating the transition dipole moment at each point by TD-DFT/ωB97XD/aug-cc-pVTZ and fitting to a polynomial in order to evaluate the second derivative with respect to Ql and Qk. In the aqueous phase the high symmetry of NO3− is broken and the first order term is no longer forbidden. However, the calculated solvated geometry still resembles the gas phase geometry and the calculated first order term does not contribute significantly to the overall oscillator strength of 1.9 × 10−6. This is a rare example of higher order vibronic coupling being more important than the lowest order term.


 Please wait while we load your content...
Please wait while we load your content...