Nanocrystallite–liquid phase transition in porous matrices with chemically functionalized surfaces†
Abstract
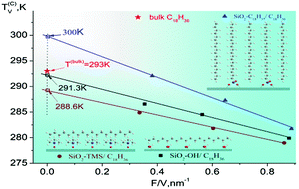
Nanocrystallite–liquid phase transitions are studied for 1-octadecene confined in the pores of chemically functionalized silica gels. These silica gels possess similar fractal geometries of the pore system but differ in chemical termination of the surface, specific surface area (F) and pore volume (V). Linear dependencies of the melting temperature and specific melting heat on the F/V ratio are found for a series of silica gels with identical surface termination. A thermodynamic model based on experimental data is established, which explains the observed shift of the phase transition parameters for porous matrices with different surface chemistries. In addition, this model allows evaluation of actual changes in nanocrystallite density, surface tension and entropy upon melting.


 Please wait while we load your content...
Please wait while we load your content...