Modulating intramolecular chalcogen bonds in aromatic (thio)(seleno)phene-based derivatives†
Abstract
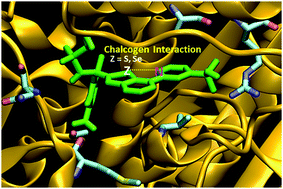
Intramolecular interactions have been proven to be the key to conformational control in drug-design. While chalcogen interactions have been shown to be present in certain ligands of the GK–GKRP target protein, in the present study, intramolecular chalcogen interactions through selenium are found to be even more promising since they form stronger interactions. Also, the flexibility/rigidity of the carbon backbone of the corresponding ligands is crucial in the conformational stability.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...