Impact of water on the BrO + HO2 gas-phase reaction: mechanism, kinetics and products†
Abstract
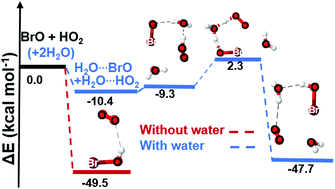
The BrO + HO2 reaction, which participates in the cycle of ozone removal via BrOH formation, was explored both in the absence and in the presence of water using ab initio calculations. Two main sets of products, (i) HBr + O3 and (ii) BrOH + O2, are formed regardless of the presence of water, following a hydrogen abstraction mechanism. The HBr + O3 products are formed from the intermediate BrOOOH adduct, whereas BrOH + O2 are formed either from the intermediate OBrOOH adduct or via a barrierless hydrogen transfer from HO2 to BrO. Owing to the formation of molecular oxygen that can bear different spin configurations, the formation of BrOH + O2 products was examined both on the singlet and the triplet surfaces. Under relevant atmospheric temperatures and pressure, the formation of products (i) is energetically and kinetically less favorable than that of products (ii). The rate coefficient at 298 K for the HBr + O3 formation was determined to be 2.00 × 10−20 cm3 molecule−1 s−1, and found to decrease by 1–2 orders of magnitude when one or both reactants are clustered with water. For the formation of BrOH + O2, a rate coefficient of 2.21 × 10−11 cm3 molecule−1 s−1 is determined on both singlet and triplet surfaces in the absence of water. Though this rate coefficient slightly decreases for the hydrated reactions, the fractions of the reactants that are effectively complexed with water are not high enough to shift the overall BrOH + O2 formation rate. The current study further indicates that humidity plays a negligible role in ozone removal via the BrO + HO2 reaction.


 Please wait while we load your content...
Please wait while we load your content...