Effect of methyl and halogen substituents on the transmembrane movement of lipophilic ions†
Abstract
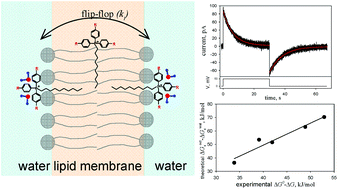
Penetrating cations are widely used for the design of bioactive mitochondria-targeted compounds. The introduction of various substituents into the phenyl rings of dodecyltriphenylphosphonium and the measurement of the flip-flop of the synthesized cations by the current relaxation method revealed that methyl groups accelerated significantly the cation penetration through the lipid membrane, depending on the number of groups introduced. However, halogenation slowed down the penetration of the analogues. This result is strictly opposite to the flip-flop acceleration observed for halogenated tetraphenylborate anions. Density functional theory and the polarizable continuum solvent model were used to calculate the solvation energies of methyltriphenylphosphonium and methyltriphenylborate analogues. A good agreement was demonstrated between the difference in the free energy of ion solvation in water and octane and the absolute value of the central free energy barrier estimated from experimental data. Our results reveal that increasing the size of the lipophilic ion can lead to both acceleration and deceleration of the transmembrane flip-flop rate depending on the substituent and sign of the ion. This finding also emphasizes the different nature of ion–water interactions for structurally similar substituted hydrophobic anions and cations.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...