Factors for the emission enhancement of dimidium in specific media such as in DNA and on a clay surface†
Abstract
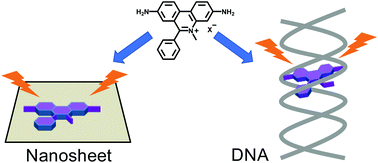
Dimidium (3,8-diamino-5-methyl-6-phenylphenanthridinium: NH2PhP) is a well-known fluorophore as a DNA probe, although its fluorescence enhancement mechanism is not clear. In this study, we investigated the fluorescence enhancement mechanism of NH2PhP on a clay surface by observing the fluorescence behavior. Four systematically selected phenanthridinium derivatives (PDs): NH2PhP, 3,8-bisdimethylamino-5-methyl-6-phenylphenanthridinium (NMe2PhP), 5-methyl-6-phenylphenanthridinium (PhP) and 5-methylphenanthridinium (P) and synthetic clay were used as guest and host materials, respectively. It was revealed that the suppression of hydrogen bonding with water (N–HOH or NH–OH2) is the dominant factor for the fluorescence enhancement on the clay surface for NH2PhP and NMe2PhP. In addition, judging from the fluorescence enhancement for NH2PhP, NMe2PhP and PhP and no fluorescence enhancement for P on the clay surface, the suppression of rotation of the phenyl ring was indicated to make a partial contribution to the fluorescence enhancement mechanism. Because the fluorescence enhancement behavior was quite similar on the clay surface and in DNA, the obtained results afford an important clue to discuss the fluorescence enhancement mechanism of NH2PhP in DNA.


 Please wait while we load your content...
Please wait while we load your content...