Electrically induced liquid–liquid phase transition in water at room temperature†
Abstract
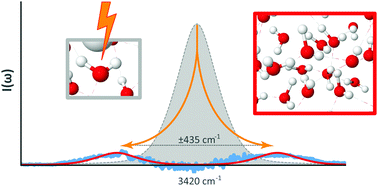
In this work we expand on findings previously reported [Wexler et al., Phys. Chem. Chem. Phys., 2016, 18, 16281] on the experimental observation of a phase transition in a hydrogen bonded liquid manifesting in long range dipole–dipole interactions. The studied system, liquid water stressed by an electric field, exhibits collective oscillations brought about through spontaneous breakdown of symmetry. Raman spectroscopy identifies the primary excitation of the emergent phase as transverse optically active phonon-like sidebands that appear on the hydrogen bonded asymmetric stretch mode. The phase transition is observed throughout the entire volume of liquid. The system also exhibits a self-similarity relation between the scattered Raman intensity and the electric field strength which further supports the conclusion that collective behavior persists against thermal disruption. The experimental findings are discussed in terms of a quantum field theory for macroscopic quantum systems.


 Please wait while we load your content...
Please wait while we load your content...