Bilayer thickness determines the alignment of model polyproline helices in lipid membranes†
Abstract
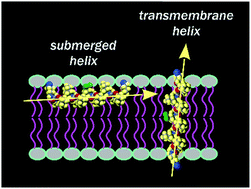
Our understanding of protein folds relies fundamentally on the set of secondary structures found in the proteomes. Yet, there also exist intriguing structures and motifs that are underrepresented in natural biopolymeric systems. One example is the polyproline II helix, which is usually considered to have a polar character and therefore does not form membrane spanning sections of membrane proteins. In our work, we have introduced specially designed polyproline II helices into the hydrophobic membrane milieu and used 19F NMR to monitor the helix alignment in oriented lipid bilayers. Our results show that these artificial hydrophobic peptides can adopt several different alignment states. If the helix is shorter than the thickness of the hydrophobic core of the membrane, it is submerged into the bilayer with its long axis parallel to the membrane plane. The polyproline helix adopts a transmembrane alignment when its length exceeds the bilayer thickness. If the peptide length roughly matches the lipid thickness, a coexistence of both states is observed. We thus show that the lipid thickness plays a determining role in the occurrence of a transmembrane polyproline II helix. We also found that the adaptation of polyproline II helices to hydrophobic mismatch is in some notable aspects different from α-helices. Finally, our results prove that the polyproline II helix is a competent structure for the construction of transmembrane peptide segments, despite the fact that no such motif has ever been reported in natural systems.


 Please wait while we load your content...
Please wait while we load your content...