Brownian dynamics assessment of enhanced diffusion exhibited by ‘fluctuating-dumbbell enzymes’†
Abstract
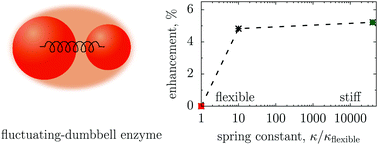
Recent experiments have reported that diffusion of enzymes can be enhanced in the presence of their substrates. Using a fluctuating dumbbell model of enzymes, it has been argued that such an enhancement can be rationalized by the reduction of the enzyme size and by the suppression of the hydrodynamically coupled conformational fluctuations, induced by binding a substrate or an inhibitor to the enzyme [Nano Lett. 2017, 17, 4415]. Herein, we critically examine these expectations via extensive Brownian dynamics simulations of a similar model. The numerical results show that neither of the two mechanisms can cause an enhancement comparable to that reported experimentally, unless very large, physically counter-intuitive, enzyme deformations are invoked.


 Please wait while we load your content...
Please wait while we load your content...