Correlation of the partial charge-transfer and covalent nature of halogen bonding with the THz and IR spectral changes†
Abstract
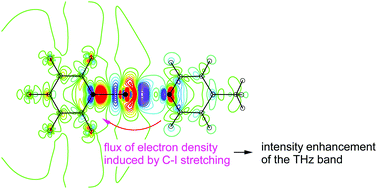
The electronic structural origin of the THz and IR spectral changes occurring upon halogen-bond formation is examined by employing the technique of electron density analysis. Theoretical calculations and analyses are conducted for the complexes of pentafluoroiodobenzene (C6F5I) and nitryl chloride (O2NCl) formed with other (halogen-bond accepting) molecules taken as typical examples. It is shown that, in the case of the C–I stretching mode of C6F5I appearing in the THz spectral region, the intensity enhancement occurring upon halogen-bond formation arises from the intermolecular charge flux and (together with the vibrational frequency) is correlated to the partial charge-transfer and covalent nature of the halogen bond. For the N–Cl stretching vibration of O2NCl, it is shown that the high-frequency shift occurring upon complex formation with NH3 arises mainly from the electrostatic effect, while the reduction of its IR intensity arises from the polarization effect and, to a larger extent, from the intermolecular charge flux. These results indicate, therefore, that there are some observable spectroscopic properties in the THz and IR region that are mainly controlled by (and, hence, shed light on) the partial charge-transfer and covalent nature of halogen bonding.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...