Determining the role of redox-active materials during laser-induced water decomposition†
Abstract
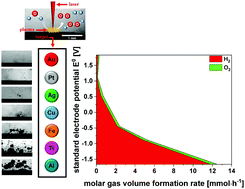
Laser ablation in liquids (LAL) drives the decomposition of the liquid inducing the formation of a large number of different redox equivalents and gases. This not only leads to shielding effects and a decrease of the nanoparticle (NP) productivity but also can directly affect the NP properties such as the oxidation degree. In this study, we demonstrate that liquid decomposition during laser ablation in water is triggered by the redox activity of the 7 different bulk materials used; Au, Pt, Ag, Cu, Fe, Ti and Al, as well as by the reactivity of water with the plasma. Laser ablation of less-noble metals like aluminum leads to a massive gas evolution up to 390 cm3 per hour with molar hydrogen to oxygen ratios of 17.1. For more noble metals such as gold and platinum, water splitting induced by LAL is the dominant feature leading to gas volume formation rates of 10 up to 30 cm3 per hour and molar hydrogen to oxygen ratios of 1.2. We quantify the material-dependent ablation rate, shielding effects as well as the amount of hydrogen peroxide produced, directly affecting the yield and oxidation of the nanoparticles on the long-time scale.


 Please wait while we load your content...
Please wait while we load your content...