Theoretical investigation on the reaction mechanism and kinetics of a Criegee intermediate with ethylene and acetylene†
Abstract
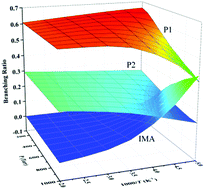
The detailed reaction mechanism of the Criegee intermediate CH2OO with ethylene and acetylene has been investigated by using the HL//M06-2X/AUG-cc-pVTZ method. The 1,3-cycloaddition of CH2OO to the unsaturated bond of ethylene or acetylene forms a five-membered ring adduct. For the reaction of CH2OO with ethylene, the subsequent ring-opening, H-shift isomerization and decomposition result in the formation of ethenol + HCHO and acetaldehyde + HCHO, and for the reaction of CH2OO with acetylene, the adduct proceeds via ring-opening and H-shift isomerization forming malonaldehyde. The calculated overall rate constant increases in the temperature range of 200–500 K, and at 298 K, it is 3.91 × 10−15 cm3 molecule−1 s−1 for the CH2OO + C2H4 reaction and 1.27 × 10−16 cm3 molecule−1 s−1 for the CH2OO + C2H2 reaction. The product branching ratio of the CH2OO + C2H4 reaction is pressure dependent, and the adduct tends to decompose to ethenol + HCHO and acetaldehyde + HCHO at lower pressures and higher temperatures. For the CH2OO + C2H2 reaction, the adduct isomerizes completely to malonaldehyde in the temperature range of 200–500 K and the pressure range of 100–1000 Torr.


 Please wait while we load your content...
Please wait while we load your content...