Mn 2p resonant X-ray emission clarifies the redox reaction and charge-transfer effects in LiMn2O4†
Abstract
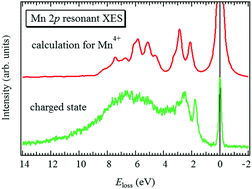
High-energy-resolution soft X-ray emission spectroscopy (XES) was applied to understand the changes in the electronic structure of LiMn2O4 upon Li-ion extraction/insertion. Mn 2p–3d–2p resonant XES spectra were analyzed by configuration-interaction full-multiplet (CIFM) calculations, which reproduced both dd and charge-transfer (CT) excitations. From the resonant XES spectra it is found that Mn3+ and Mn4+ coexist in the initial state, while this changes into Mn4+ in the charged-state. For the discharged-state, the Mn3+ component appears again although the dd excitations are slightly modified from those for the initial state. Furthermore, negative CT energy is expected for the Mn4+ configuration, which suggests very strong hybridization between the Mn 3d and O 2p orbitals. The large difference in the CT effect between the Mn4+ and Mn3+ states should give mechanical stress to the Mn–O bond during charge–discharge cycling, leading to capacity fading.


 Please wait while we load your content...
Please wait while we load your content...