Carbonization of transition metals in molten salts†
Abstract
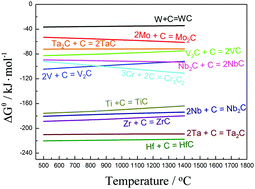
The carbonization of transition metals in molten salts was performed to study the effect of electrochemical polarization and molten salt medium on the carbonization process. The carbonization of niobium (Nb) has been systemically investigated in Ar atmosphere and molten salts. The effect of particle size and structure of the starting materials, molten salt medium, and electric field on the formation of NbC was studied to reveal the dynamic barriers in the carbonization process. A native oxide layer and/or niobate derivatives generated on the Nb particles are the main barriers for the mass transfer of carbon and Nb towards each other. The results showed that molten salts can accelerate the formation of NbC by enhancing the diffusion of carbon, and the kinetic barrier can be effectively eliminated by supplying negative polarization to the cathode in molten salts to remove the oxide barriers, thereby enhancing carbonization. Besides Nb, tungsten (W), molybdenum (Mo), titanium (Ti), and tantalum (Ta) can also be carbonized in molten salts with the assistance of an applied electric field.


 Please wait while we load your content...
Please wait while we load your content...