Mechanistic insight into E22Q-mutation-induced antiparallel-to-parallel β-sheet transition of Aβ16−22 fibrils: an all-atom simulation study†
Abstract
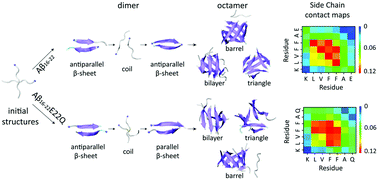
Alzheimer's disease is associated with the abnormal self-assembly of amyloid-β (Aβ) peptide into toxic oligomers and fibrils. Recent experiments reported that Aβ16−22, containing the central hydrophobic core (CHC) of Aβ, formed antiparallel β-sheet fibrils, while its E22Q mutant self-assembled into parallel β-sheet fibrils. However, the molecular mechanisms underlying E22Q-mutation-induced parallel β-sheet fibril formation are not well understood. Herein, we performed molecular dynamics (MD) simulations to study the dimerization processes of Aβ16−22 and Aβ16−22E22Q peptides. β-Sheet dimers with diverse hydrogen bond arrangements were observed and they exhibited highly dynamic and interconverting properties. An antiparallel-to-parallel β-sheet transition occurred in the assembly process of the E22Q mutant, but not in that of Aβ16−22. During this conformational transformation process, the inter-molecular Q22–Q22 hydrogen bonds were first formed and acted as a binder to facilitate the two chains forming a parallel orientation, then the hydrophobic interactions between residues in the CHC region consolidated this arrangement and drove the main-chain H-bond formation, hence resulting in parallel β-sheet formation. However, parallel β-sheets were less populated than antiparallel β-sheets of Aβ16−22E22Q dimers. In order to explore whether parallel β-sheets became dominant in larger size oligomers, we investigated the conformational ensembles of Aβ16−22 and Aβ16−22E22Q octamers by conducting replica exchange molecular dynamics (REMD) simulations. The REMD simulations revealed that the population of parallel β-strand alignment increased with an increase of the size of ordered Aβ16−22E22Q β-sheet oligomers, implying that the formation of full parallel β-sheets requires larger sized oligomers. Our findings provide a mechanistic explanation for the E22Q-mutation-induced formation of parallel β-sheet fibrils observed experimentally.


 Please wait while we load your content...
Please wait while we load your content...