Electronic structure and high-temperature thermochemistry of BaZrO3−δ perovskite from first-principles calculations†
Abstract
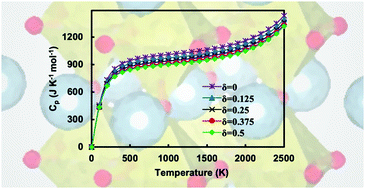
ABO3−δ perovskites are attractive candidates for high-temperature mixed ionic electronic conduction processes, due to their ability to produce mixed oxidation states and accommodate oxygen vacancies. Here, we examine the electronic structure and high-temperature thermochemistry of stoichiometric and non-stoichiometric cubic BaZrO3−δ perovskites for high defect concentration (δ = 0–0.5) using first-principles density functional theory (DFT) and density functional perturbation theory (DFPT) calculations. Our results show that the electronic structures of these perovskites under increasing oxygen deficiency are characterized by highly localized reduction of Zr4+ t2g orbitals in the vicinity of the oxygen defects, irrespective of the value of δ. Temperature dependent thermodynamic properties of pristine- and defective-BaZrO3−δ show consistency with oxygen vacancy concentration. A comparison of predicted thermochemical properties with and without explicit vibrational corrections demonstrates their relative stability and implications at high-temperatures, as reduction Gibbs free energies in BaZrO3−δ exhibit large deviations above 1000 K. We elucidate the physical origins of these deviations via a phonon mode analysis.


 Please wait while we load your content...
Please wait while we load your content...