Non-equilibrium thermodynamics as a tool to compute temperature at the catalyst surface
Abstract
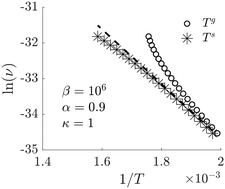
The surface temperature is computed for a heterogeneous catalytic reaction model, namely the oxidation of carbon monoxide on platinum. The surface temperature was found using non-equilibrium thermodynamic theory, a theory which provides the proper dependencies between heat and mass fluxes and the reaction rate. The theory predicts a possible coupling between the reaction rate and the thermal driving force and can help extend classical reaction kinetics. In the absence of direct measurements, we explore the coupling numerically. The results are able to capture experimental data reported in the literature, and give new insights into why Arrhenius plots may turn out to be non-linear.


 Please wait while we load your content...
Please wait while we load your content...