Optical band gap of cross-linked, curved, and radical polyaromatic hydrocarbons†
Abstract
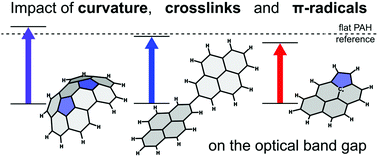
In this work, the optical band gaps of polycyclic aromatic hydrocarbons (PAHs) crosslinked via an aliphatic bond, curved via pentagon integration and with radical character were computed using density functional theory. A variety of different functionals were benchmarked against optical band gaps (OBGs) measured by ultraviolet-visible spectroscopy with HSE06 being most accurate with a percentage error of 6% for a moderate basis set. Pericondensed aromatics with different symmetries were calculated with this improved functional providing new scaling relationships for the OBG versus size. Further calculations showed crosslinks cause a small decrease in the OBG of the monomers which saturates after 3–4 crosslinks. Curvature in PAHs was shown to increase the optical band gap due to the resulting change in hybridisation of the system, but this increase saturated at larger sizes. The increase in OBG between a flat PAH and a strained curved one was shown to be equivalent to a difference of several rings in size for pericondensed aromatic systems. The effect of σ-radicals on the optical band gap was also shown to be negligible, however, π-radicals were found to decrease the band gap by ∼0.5 eV. These findings have applications in understanding the molecular species involved in soot formation.


 Please wait while we load your content...
Please wait while we load your content...