An ab initio based full-dimensional potential energy surface for OH + O2 ⇄ HO3 and low-lying vibrational levels of HO3†
Abstract
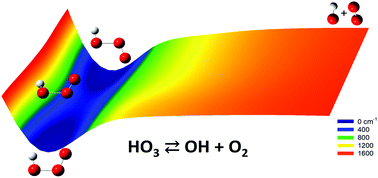
To provide an in-depth understanding of the HO3 radical and its dissociation to OH + O2, a six-dimensional potential energy surface (PES) has been constructed by fitting 2087 energy points for the electronic ground state of HO3 (X2A′′) using the permutation invariant polynomial-neural network (PIP-NN) approach. The energy points were calculated using an explicitly-correlated and Davidson-corrected multi-reference configuration interaction method with the correlation-consistent polarized valence double zeta basis (MRCI(Q)-F12/VDZ-F12). On the PES, the trans-HO3 isomer is found to be the global minimum, 33.0 cm−1 below the cis-HO3 conformer, which is consistent with previous high-level theoretical investigations. The dissociation to the OH + O2 asymptote from both conformers is shown to be barrierless. As a benchmark from a recently developed high-accuracy thermochemistry protocol, D0 for trans-HO3 is calculated to be 2.29 ± 0.36 kcal mol−1, only slightly deeper than the value of 2.08 kcal mol−1 obtained using the PES, and in reasonable agreement with the experimentally estimated value of 2.93 ± 0.07 kcal mol−1. Using this PES, low-lying vibrational energy levels of HO3 are determined using an exact quantum Hamiltonian and compared with available experimental results.


 Please wait while we load your content...
Please wait while we load your content...
