Cation pair formation in copper and palladium exchanged MFI zeolite frameworks – a theoretical study†
Abstract
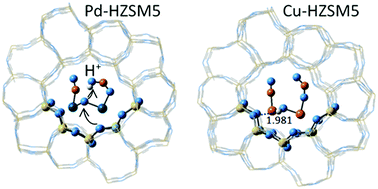
The coordination of copper and palladium cations in the channels of MFI frameworks is examined by the QM/MM approach within the ONIOM method. Density functional theory is applied to the high layer of the ONIOM models, and different rings and ring associations are considered with the aim to find the optimal positions for cation pair formation. The Al atoms were located at tetrahedral sites which are known to have high occupancy factors from crystal structure studies. Divalent cations have a strong preference towards six-member ring coordination, and while the energy differences between five-member ring coordination and six-member ring coordination are not that large for the monovalent cations and hydroxyl-cations (M+ and M(OH)+, M = Cu, Pd) they still prefer six-member ring coordination in the straight channel. The monovalent cations form dimers [Pd–Pd]2+ and [Cu–Cu]2+ with bond lengths of 2.50–2.57 Å, but while palladium cations form dimers independent of the Si, Al ordering, the copper cation dimers are stable only with closely positioned negative framework charges. Separation of the two Cu+ cations at distances >4 Å is preferred, unless an oxygen bridge is formed. The cation dimers are exposed in the intersection volume of the straight and sinusoidal channels of MFI, which greatly favors the accessibility of reactants. The oxygen bridged Pd cations accept protons from Brønsted acid sites to form Pd–OH+–Pd and they are able to transfer this proton to reactants: upon co-adsorption of NO and NO2 on Pd–OH+–Pd, protonation of NO2 occurs. In contrast, Cu–OH+–Cu groups reach stabilization by forming hydrogen bonds to framework oxygen atoms. [Pd–Pd] cation dimers alone, or in oxygen-bridged form, provide an energetically favorable route for NO dissociation. Two different configurations with wide and narrow angle ∠MOM were examined for partial oxidation of methane to methanol. The narrow angle [Cu–O–Cu] was found to ease methanol desorption. The active site [Pd–O–Pd] is superior to [Cu–O–Cu], providing lower energy barriers for methane activation and methanol desorption.


 Please wait while we load your content...
Please wait while we load your content...