Surface thermodynamics of silicate compounds: the case of Zn2SiO4(001) surfaces and thin films
Abstract
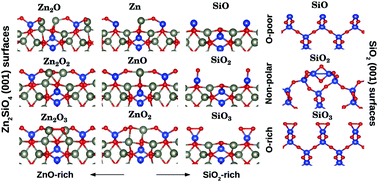
Silicate compounds are ubiquitous in nature and display a vast variety of structures and properties. Thin silicate films may also form under specific conditions at interfaces between metals and silica. In the present study, we focus on zinc silicate and present a thorough density functional theory-based study of polar and non-polar (001) surfaces of various stoichiometry of its tetragonal polymorph t-Zn2SiO4. At the non-polar surfaces, the main features are the existence of the chain reconstruction at the ZnO termination, and the presence of unsaturated surface silanols at the SiO2 termination. Stabilization of polar surfaces is provided by the formation of O22− peroxo groups, reduction of the surface or subsurface Si atoms or formation of Zn22+ groups, depending upon the surface stoichiometry. While the non-polar stoichiometric and ZnO rich terminations are the most stable in a large part of the accessible phase diagram, the SiO2 termination is less stable due to the absence of siloxane group formation. We show that, while bulk Zn2SiO4 is stable with respect to decomposition into the ZnO and SiO2 oxides, the same is not true for ultra-thin films due to the fundamental difference of silicate and silica surface energies. Preliminary results show that a similar conclusion could be drawn for Fe2SiO4. This study opens the way towards a deeper understanding and possible improvement of zinc adhesion at silica surfaces, of crucial industrial importance.


 Please wait while we load your content...
Please wait while we load your content...