Pressure induced multi-centre bonding and metal–insulator transition in PtAl2†
Abstract
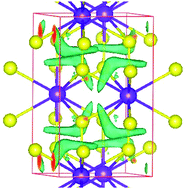
A study on pressure induced metallization or metal–insulator transition (MIT) of materials is of enormous importance in high pressure research not only in view of various possible device applications but also for basic physical insight into their mechanism. Though the former transition (metallization) is quite common, the latter one (MIT) is comparatively rare, and only a few simple metals (Na, Al, etc.) are predicted to be an insulator at megabar pressure. In this work, we have shown that the binary intermetallic compound (PtAl2) exhibits a pressure induced metal–insulator transition comparatively at low pressure, near ∼28 GPa. By means of first principles calculations, we have established that a unique multi-centre bonding is developed under pressure which causes charge-density-ordering in the system and finally leads to MIT and structural phase transition involving unit cell reduction. In search of some exotic properties, we have found that the system has a good thermoelectric figure-of-merit in its insulating phase.


 Please wait while we load your content...
Please wait while we load your content...