An elevated concentration of MoS2 lowers the efficacy of liquid-phase exfoliation and triggers the production of MoOx nanoparticles†
Abstract
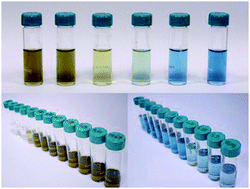
It is generally accepted that liquid-phase exfoliation (LPE) enables large-scale production of few-layer MoS2 flakes. In our work, we studied in detail few-layer MoS2 oxidation in the course of standard LPE in a water/ethanol solution. We demonstrate that an increase of the initial MoS2 concentration above a certain threshold triggers a pronounced oxidation and the exfoliation process starts to produce MoOx nanoparticles. A subsequent decrease of the water pH along with an increased content of SO42− suggests an oxidation scenario of few-layer MoS2 oxidation towards MoOx nanoparticles. Moreover, the lowered pH leads to agglomeration and sedimentation of the few-layer MoS2 flakes, which significantly lowers their production yield. We employed a large number of physico-chemical techniques to study the MoS2-to-MoOx transformation and found a threshold value of 10 mg ml−1 of the initial MoS2 concentration to trigger this transformation.


 Please wait while we load your content...
Please wait while we load your content...