Thermodynamic evidence of a transition in ZIF-8 upon CH4 sorption†
Abstract
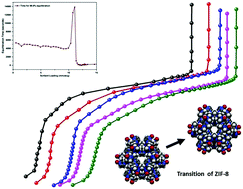
We present the results of an experimental study of methane sorption in ZIF-8. We measured isotherms at five different temperatures between 87 K and 107 K. We have observed three sub-steps in each of the isotherms. The intermediate sub-step had not been observed experimentally in previous studies of this system. This newly determined experimental feature suggests that a transition is taking place in the sorbed system (this newly observed sub-step occurs over a loading interval where published computer simulation results for CH4 in ZIF-8 had identified a structural transition occurring in the sorbent). We have studied the kinetics of adsorption for this system (we measure the time required for the system to reach equilibrium after gas is added to the experimental cell as a function of sorbent loading). We observed a sharp peak in the equilibration time at high loadings, below saturation. We have explored the isosteric heat of adsorption, and its dependence on sorbent loading, for this system. We found a broad peak in the isosteric heat at loadings corresponding to the intermediate isotherm sub-step. Previously reported computer simulations for the isosteric heat dependence on loading for CH4 in ZIF-8 are in good agreement with our experimental results for this quantity.


 Please wait while we load your content...
Please wait while we load your content...