Convergent energies and anharmonic vibrational spectra of Ca2H2 and Ca2H4 constitutional isomers†
Abstract
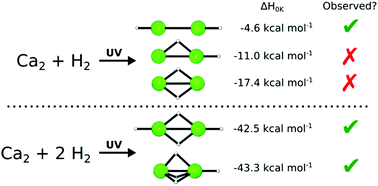
Three constitutional isomers of both Ca2H2 and Ca2H4 have been characterized with molecular electronic structure theory. Correlation methods as complete as CCSDT(Q) and basis sets as large as cc-pwCV5Z have been used to converge the relative energies within chemical accuracy (≤1 kcal mol−1). Anharmonic vibrational frequencies were computed using second-order vibrational perturbation theory employing CCSD(T)/cc-pwCVTZ cubic and quartic force-fields and a CCSD(T)/cc-pwCVQZ quadratic force field. The monobridged [Ca(μ2-H)CaH] and dibridged [Ca(μ2-H)2Ca] isomers of Ca2H2 were predicted to lie 6.5 and 12.9 kcal mol−1 below the energy of the classical HCaCaH linear isomer, respectively. Despite the energetic favorability of the bridged Ca2H2 isomers, we conclude (surprisingly) that only the higher energy linear structure has been observed in the laboratory. At 0 K, the tribridged [Ca(μ2-H)3CaH] isomer of Ca2H4 is predicted to be enthalpically favored by 0.9 kcal mol−1 in comparison to the enthalpy of the dibridged [HCa(μ2-H)2CaH] structure. Comparison of experiment with our computed frequencies suggests that the observed vibrational features arise from both the dibridged and the tribridged Ca2H4 structures.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
