Computer simulations of the adsorption of an N-terminal peptide of statherin, SN15, and its mutants on hydroxyapatite surfaces†
Abstract
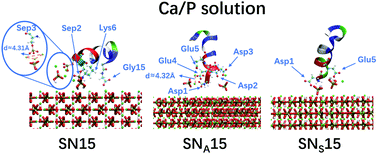
Statherin is a 43 amino acid long protein, which plays an important role in the process of biomineralization of enamel. In this work, we investigated the solvent effect on the adsorption of a peptide from the N-terminus of statherin, SN15, and its mutants SNA15 and SNS15 on the (001) face of hydroxyapatite [Ca10(PO4)6(OH)2, or HAP] with molecular dynamics simulations. The simulation results showed that the adsorption of the three peptides onto the HAP(001) surface was primarily driven by salt-bridge and electrostatic attraction in calcium phosphate (Ca/P) and sodium chloride (NaCl) solutions, respectively. SN15 adsorbs on the HAP surface with the strongest electrostatic interaction, while SNS15 is the weakest. Besides, Ca2+ around SN15 can form an equilateral triangle, which resembles the structure formed by Ca(2) ions in the HAP(001) crystal face, and this looks like the initial stage of HAP nucleation. The conformational changes of SN15 on HAP are analyzed by the root-mean-square deviation. It shows that SN15 is more stable in Ca/P solution while SNS15 is more stable in NaCl solution; the stability of SNA15 is almost the same in both solutions. This work reveals the adsorption mechanism of a series of SN peptides on the HAP surface and provides guidelines for the design of biomaterials for restoring etched enamel and regulating biomineralization.


 Please wait while we load your content...
Please wait while we load your content...